Shanza Kiran1, Snovia Ahmed1, Abdul Basit2, Khurram Shahzad3, Asma Hayat4, Muhammad Zeeshan Riaz5, Muhammad Haris Ali Khan6, Shahzad Bashir1*
1School of Biochemistry, Minhaj University, Lahore, Pakistan.
2Department of Horticulture, Kyungpook National University, 41566 Daegu, South Korea.
3Department of Chemistry, University of Sahiwal, Pakistan.
4Dr. M. A. Kazi Institute of Chemistry, University of Sindh, Jamshoro -76080, Pakistan.
5Department of biochemistry, KFUEIT, Rahim yar khan, Pakistan.
6Sheikh Zayed Medical College, Rahim yar khan, Pakistan.
ABSTRACT
The revival of natural dyes in different walks of life is due to stringent environmental standards imposed by many associations. In this current study, rose petals were selected for dyeing cotton fabrics under the influence of microwave radiation. For isolation of colorant (anthocyanin) in aqueous, acidic alkaline and organic media from rose petals, microwave radiation for 1, 2, 3, 4, 5, and 6 min was given. These treated and untreated extracts were employed to dye the irradiated and un-irradiated fabrics. Different dyeing variables such as temperature, time, pH, salt as well as volume of dye bath were optimized using optimal extraction conditions. For introduction of new shades and improvement of colorfastness properties, different concentrations of chemical mordants such Al and Fe and bio-mordants such as extracts of henna and turmeric were employed. All dyed fabrics were subjected to CIE LAB System for evaluation of K/S and L, a, b values using Spectra flash SF600. To observe the influence of radiation treatment on colorfastness characteristics, suggested standard methods of ISO for colorfastness to light and washing rubbing were rated. Finally, the results obtained from various parameters were analyzed statistically.
Keywords: Rosa indica, Dyes, cotton dyes, Curcumin, Henna, Microwave Assisted Extraction
Introduction
Plants are the major source of modern medicines. Anthocyanins are present in high concentrations in rose petals which gave assertion to these signs because anthocyanins have anti-inflammatory, antiseptic, and antioxidant activity. It also gave strength to the plant’s vascular system and decreases blood platelet stickiness [1]. Rose is psychologically economically, clinically, and scientifically very important. Rosehip also contains coheres, amino acids, carotenoids, and fruit acids. Fatty oil is also produced from its seeds which are used to cure dermatitis which is the skin allergy flowers and especially the petals give physical protection to the reproductive organs [2]. Rose oil which is obtained from rose petals is steam distilled by devastating. The byproduct is rose water which is obtained after steam distillation; it acts as an excellent soothing agent. The genus Rosa contains over 130 documented species. Roses are inhabitants of the Northern Hemisphere. It naturalized worldwide. Three subgenera (Platyrhodon, Eurosa, and Hesperhodos) are there. Amongst, coloring flowers, roses (Rosa Indica) are an excellent source of reddish pink to greenish natural dye for dyeing the fabric. Having 150 species around the world, this flower is called the queen of flowers due to its beautiful look [3].
Dyes are coloring organic compounds which develop color to items either paper, fiber, or anything else in an aqueous solution. Dyes are utilized in collaboration with a mordant to give better coloring characteristics, offering combinations of soothing, soft, and shining color shades [4]. Dyes fix themselves to the substance via adsorption, ionic or covalent bonds to the substance by mechanical force. Dyes should be sustainable, and compatible, having the capability to fix on the substance firmly [5]. Dyes must have an appropriate color. Natural dyes are being applied at a very vast level due to color fixation and color strength, proving themselves as a basic tool in the field of textiles [6].
Natural dyes not only provide anti-oxidant, anti-inflammatory, anti-bacterial, and anti-cancer characteristics but also produce beautiful and soothing shades. These dyes are ecologically having no side effects on the skin and no disposal problem [7]. These dyes are non-allergic, easily biodegradable, and compatible with the eco-system [8] and the use of natural dyes minimizes the toxic waste and risk to human health [9]. Microwaves are electromagnetic radiations of frequency ranging from 103MHz to 106 MHz These are non-ionizing radiations and a powerful source of non-contact heating Only those molecules of fabric can absorb microwave radiations that have permanent dipole moment because their frequency resonates with the frequency of microwave radiations [10].
Many processes are under implementation process but on the view of cost, time, energy, and effectiveness, the radiation-induced extraction process has been recently gaining widespread popularity. One of these is the microwave-assisted extraction (MAE) technique which yields better quality extracts in higher amounts as it involves less energy and a simple technique of faster start-up and shut-down times. Microwaves are electromagnetic radiations that heat volumetrically and selectively. Volumetric heating permits the system to be heated rapidly while in selective heating thermal gradient is created [11]. Microwave heating is very rapid and uniform resulting in an energy and time-saving process. Another advantage is reduced size of equipment and waste, controlled heating process, and no direct interaction between the source and materials [12]. Microwave extraction depends upon the nature of the solvent, volume, extraction temperature, time, microwave power, and properties of the product. Microwaves are environment-friendly sources of radiation which enhanced the dye uptake ability of fabrics [13].
Material and Methods
Collection of Materials
Rose (Rosa indica) was collected from the Botanical Garden of Lahore, Pakistan. Petals of rosa indica were washed with deionized water and dried at room temperature. Dried petals were ground finely and put through a sieve of 20 mesh size to get powder of homogeneous particle size. A piece of cotton fabric was purchased from a local textile market in Lahore, Pakistan. All chemicals and reagents used in our experiments such as Hydrochloric Acid, Sodium Hydroxide, and sodium chloride were obtained from E-Merck Darmstadt, Germany. For bio-mordant leaves of henna and rhizome of turmeric were purchased from the market.
Extraction and irradiation process
For microwave treatment, powder, extracts, and cotton fabric were irradiated for 1-6 min at high power using a commercially available orient oven. Three media such as aqueous, basic, and acidic were utilized to extract natural colorants from the irradiated and un-irradiated powder. The extract has been irradiated for up to 6 minutes by using a microwave oven at 600C. The aqueous extract was prepared by boiling petals powder (10g) with water (100 mL) for 40-minute keeping (M: L, 1:25). After boiling, the material was filtered and the filtrate obtained was microwave treated for 1-6 min. The Patels powder (10 g) was boiled with an acidic solution of 3% HCl by keeping (M: L, 1:25). Then the extract was filtered and the acidic extract and cotton fabric were exposed to microwave radiation for up to 6 minutes. Then dyeing of fabrics was done. The Rose petals powder (10g) was boiled with a basic solution of 2% Na2SO4 by keeping (M: L, 1:25). Then the extract was filtered two times and the basic extract and cotton fabrics were exposed to microwave radiation for up to 6 minutes. Then dyeing of fabrics was done.
Optimization of different dyeing parameters
Various dyeing parameters were optimized by changing the amount of powder, temperature salt concentration (NaCl), pH, and time. The optimal fabrics were dyed at different temperatures such as 20, 40, 60, 80, and 90 0C by using time intervals of 20, 30, 40, 50, and 60 minutes using a dye bath of 1-7 pH and (M: L; 1:25). To achieve maximum exhaustion, 1-10 % of table salt was employed using a dye bath of 10-70 mL by using acidified methanolic extract to optimize powder 2, 4, 6, 8, and 10 g of rose petals [14]. To improve the color fastness and color strength properties of natural dyes chemical mordants such as Fe (iron sulphate) and Al (potash alum) and bio-mordant such as turmeric (curcumin) and henna(lawsone)were used as post mordanting and pre mordanting at optimal conditions. The crude powders of respective plants were boiled with distilled water for 1 h keeping the material-to-liquor ratio of 1:25 For extraction of bio-mordants from plant sources.
Evaluation of characteristics of dyed and undyed fabrics
The Color strength, L, a, and b values of all dyed fabrics were investigated in the CIE Lab system using Spectra flash SF 600 with an illuminant of D 65 100 Observer at Govt. College University Faisalabad Pakistan. Colorfastness properties of the optimum dyed fabrics were evaluated using ISO standard method. A Crock meter was used for calculating colorfastness to rubbing. The change in color of the dyed fabric was evaluated by using a grayscale of ISO/AATCC.
RESULTS AND DISCUSSION
Aqueous Media as Extraction Solvent
The aqueous extraction method is cheap, eco-friendly, and easy to perform and also enhances the efficiency of the extraction process. The results in Fig. 1 showed that microwave treatment of aqueous for 4 minutes gave excellent color strength on the irradiated cotton with a high K/S value. But higher irradiation time showed lower K/S values. That’s why optimal irradiation time is an important factor in the extraction and dyeing process. Short irradiation time does not break the cell wall and also does not mix the colorant well into the solvent. But in case of higher irradiation time, other phytochemicals may evolve which can interfere with the coloration process [15]. Hence it is recommended to irradiate the aqueous extract for 4 minutes to obtain good color strength on irradiated fabrics. Lab values in Table 1 revealed that the sample is brighter (L*=67.46), less red (a* = 3.83), and yellow (b* = 12.25) for 4 minutes of irradiation. But in the case of non-irradiated extract (NRE)/ non-irradiated cotton fiber (NRCF), the sample is darker (L* = 58.62), redder (a* = 12.24), and more yellow (b* = 25.27). So, it is concluded that the aqueous extract should be irradiated for 4 minutes to dye the irradiated cotton fiber to get excellent color strength.
Table 1: color coordinates of cotton fabric dyed with aqueous extract of rose petals
| Microwave time(min.) | Sample name | L* | a* | b* |
| 1 | NRE/NRCF | 68.43 | 7.98 | 20.13 |
| NRE/RCF | 67.52 | 7.76 | 19.23 | |
| RE/NRCF | 63.89 | 5.29 | 14.57 | |
| RE/RCF | 63.16 | 11.32 | 18.92 | |
| 2 | NRE/NRCF | 65.29 | 6.23 | 16.65 |
| NRE/RCF | 64.56 | 10.23 | 21.17 | |
| RE/NRCF | 65.97 | 6.38 | 15.83 | |
| RE/RCF | 64.21 | 5.27 | 14.34 | |
| 3 | NRE/NRCF | 63.17 | 9.75 | 22.17 |
| NRE/RCF | 62.29 | 6.37 | 16.03 | |
| RE/NRCF | 63.75 | 5.13 | 14.14 | |
| RE/RCF | 61.25 | 6.93 | 16.22 | |
| 4 | NRE/NRCF | 58.62 | 12.24 | 25.27 |
| NRE/RCF | 69.34 | 5.57 | 15.39 | |
| RE/NRCF | 61.28 | 6.20 | 17.28 | |
| RE/RCF | 67.46 | 3.83 | 12.25 | |
| 5 | NRE/NRCF | 61.54 | 10.93 | 22.43 |
| NRE/RCF | 70.86 | 7.03 | 15.97 | |
| RE/NRCF | 63.14 | 5.92 | 16.06 | |
| RE/RCF | 68.57 | 5.88 | 14.98 | |
| 6 | NRE/NRCF | 64.75 | 7.15 | 16.07 |
| NRE/RCF | 63.21 | 10.84 | 16.32 | |
| RE/NRCF | 64.20 | 4.70 | 15.13 | |
| RE/RCF | 64.70 | 7.13 | 16.83 |
RE: Irradiated Extract NRE: Non-irradiated Extract RCF: Irradiated Cotton Fabric
NRCF: Non-irradiated Cotton Fabric
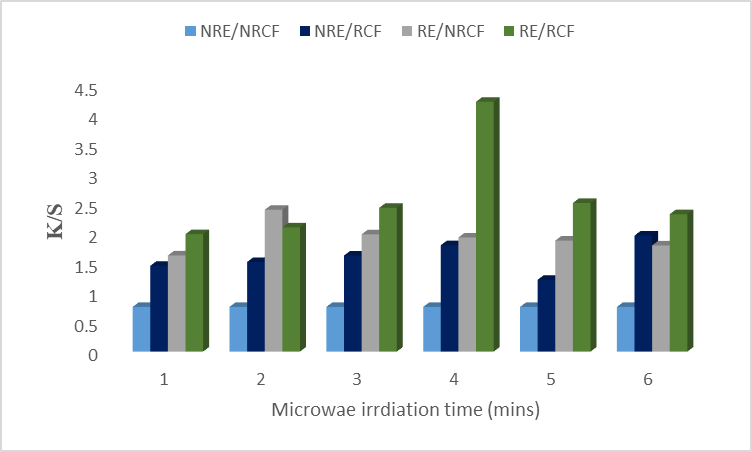
Fig. 1: Effect of aqueous extract on dyeing of cotton fabrics using rose petals extract
Acidic Extract
In addition to microwave radiations, the medium of the dye bath is also an important factor in the extraction and dying process to get efficient results. The results showed that rose petals extract gave excellent color strength to the cotton fiber by using acidic conditions. A previous study showed that maximum color strength was obtained by using an acidic medium to dye cotton fiber because it stabilize the ionic or covalent bindings between the hydroxyl group of dye and the amino group of cotton [16]. Fig. 2 revealed that an acidic extract of rose petals irradiated for 4 minutes give excellent color strength with a darker shade and show higher K/S values. The optimum irradiation time is required to get the maximum results. An acidic medium increases the dyeing efficiency of the extract because it provides active protons. At the low and higher times, the irradiated extract showed low K/S values on irradiated cotton fiber. Table 2 shows the Lab values which revealed that the sample is brighter (L* = 59.24), redder (a* = 12.54), and more yellow (b* = 26.73) for 4 minutes of irradiation. So, it is recommended to irradiate the acidic extract for 4 minutes to get excellent color strength on the irradiated cotton fiber.
Table 2: Color coordinates of cotton fabric dyed with acidic extract of rose petals
| Microwave time(min.) | Sample name | L* | a* | b* |
| 1 | NRE/NRCF | 74.67 | 9.51 | 25.58 |
| NRE/RCF | 73.27 | 6.23 | 21.84 | |
| RE/NRCF | 70.66 | 7.62 | 24.09 | |
| RE/RCF | 66.13 | 10.04 | 26.47 | |
| 2 | NRE/NRCF | 66.34 | 10.43 | 27.02 |
| NRE/RCF | 71.30 | 7.09 | 23.15 | |
| RE/NRCF | 72.58 | 7.83 | 25.14 | |
| RE/RCF | 73.93 | 8.23 | 25.51 | |
| 3 | NRE/NRCF | 72.75 | 7.16 | 22.54 |
| NRE/RCF | 75.01 | 5.04 | 19.39 | |
| RE/NRCF | 71.03 | 6.71 | 25.56 | |
| RE/RCF | 74.64 | 6.13 | 23.14 | |
| 4 | NRE/NRCF | 68.48 | 8.76 | 23.03 |
| NRE/RCF | 67.53 | 10.35 | 25.73 | |
| RE/NRCF | 60.59 | 10.27 | 24.07 | |
| RE/RCF | 59.24 | 12.54 | 26.73 | |
| 5 | NRE/NRCF | 65.14 | 10.27 | 22.82 |
| NRE/RCF | 67.11 | 11.59 | 24.22 | |
| RE/NRCF | 68.77 | 8.95 | 23.08 | |
| RE/RCF | 63.92 | 11.76 | 26.34 | |
| 6 | NRE/NRCF | 63.13 | 12.97 | 20.99 |
| NRE/RCF | 64.79 | 10.53 | 23.36 | |
| RE/NRCF | 70.32 | 7.91 | 22.43 | |
| RE/RCF | 65.07 | 11.29 | 21.73 |
RE: Irradiated Extract NRE: Non-irradiated Extract RCF: Irradiated Cotton Fabric
NRCF: Non-irradiated Cotton Fabric
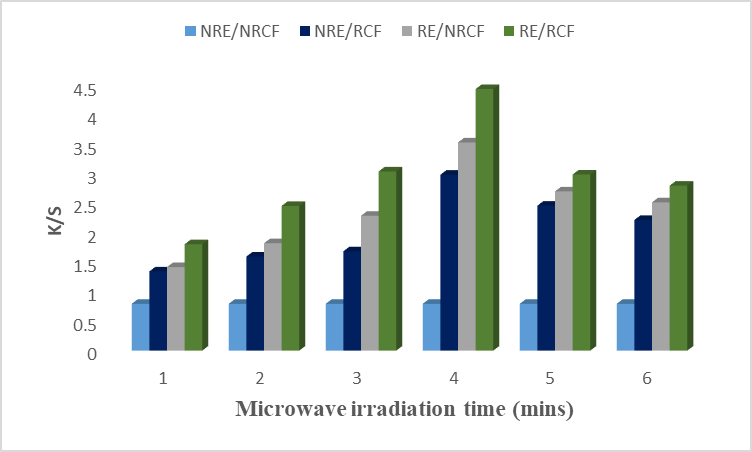
Fig. 2: Effect of acidic extract on dyeing of cotton fabrics using rose petals extract.
Basic Extract
The basic medium disturbs the dyeing behavior of the colorant. Results given in Fig. 3 revealed that samples in basic medium show low color strength with low K/S values. Microwave irradiation does not physiologically affect the extract and cotton fiber. It is also found that microwave treatment does not affect the ability of rose petals alkaline extract to much extent. The reason for low K/S values may be that the colorant of the rose petals is acidic and may lose stability due to neutralization when extracted with base. It is suggested to use rose petals basic extract irradiated for 4 minutes to dye the irradiated cotton fibers. Lab values showed that the sample is lighter (L* = 67.88), redder (a* = 8.09), and more yellow (b* = 18.03) It is found that by employing different extracts irradiated at different times, aqueous and acidic extracts show higher K/S values for irradiated cotton for 4 minutes. The acidic extract showed excellent results with the highest K/S values at 4 minutes. But the extract in basic conditions did not show good results as compared to the acidic and aqueous medium. Hence, the acidic extract irradiated for minutes should be used for the further dyeing process.
Table 3: Color coordinates of cotton fabrics dyed with basic extract of rose petals
| Microwave time(min.) | Sample name | L* | a* | b* |
| 1 | NRE/NRCF | 67.13 | 11.47 | 16.08 |
| NRE/RCF | 72.04 | 9.04 | 15.21 | |
| RE/NRCF | 71.06 | 13.58 | 24.73 | |
| RE/RCF | 57.13 | 9.63 | 15.92 | |
| 2 | NRE/NRCF | 75.92 | 10.52 | 20.37 |
| NRE/RCF | 68.19 | 10.92 | 17.14 | |
| RE/NRCF | 68.11 | 7.23 | 16.03 | |
| RE/RCF | 70.69 | 10.77 | 17.83 | |
| 3 | NRE/NRCF | 72.97 | 6.18 | 16.32 |
| NRE/RCF | 74.07 | 6.23 | 16.19 | |
| RE/NRCF | 72.26 | 10.07 | 18.27 | |
| RE/RCF | 72.98 | 9.14 | 15.32 | |
| 4 | NRE/NRCF | 66.42 | 12.49 | 19.37 |
| NRE/RCF | 68.21 | 11.09 | 19.02 | |
| RE/NRCF | 71.89 | 9.86 | 16.87 | |
| RE/RCF | 67.88 | 8.09 | 18.03 | |
| 5 | NRE/NRCF | 72.14 | 9.96 | 18.17 |
| NRE/RCF | 70.48 | 11.34 | 19.92 | |
| RE/NRCF | 66.12 | 13.74 | 21.97 | |
| RE/RCF | 63.17 | 13.99 | 21.29 | |
| 6 | NRE/NRCF | 65.22 | 12.96 | 20.13 |
| NRE/RCF | 74.15 | 6.25 | 18.02 | |
| RE/NRCF | 72.76 | 9.53 | 19.23 | |
| RE/RCF | 57.92 | 14.97 | 24.79 |
RE: Irradiated Extract NRE: Non-irradiated Extract RCF: Irradiated Cotton Fabric
NRCF: Non-irradiated Cotton Fabric
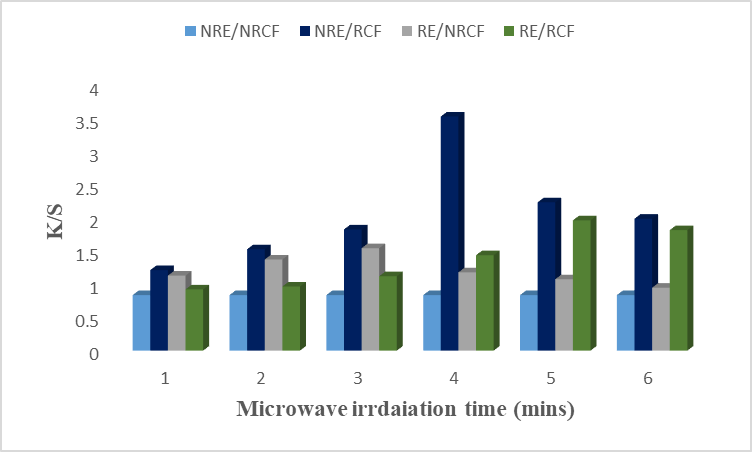
Fig. 3: Effect of basic extract on dyeing of cotton fabrics using rose petals
Powder Amount optimization
Optimization of powder amount is necessary because it plays a major role in the coloration process of cotton fiber. A low amount of colorant is extracted from a low amount of powder while a higher amount of powder causes the evolution of other phytochemicals which can disturb the colorant process. Fig 4 shows that 4 g of powder gives excellent color strength with higher K/S values. Above and below this optimal amount of powder, color strength, and K/S values decreased. Lab values given in Table 4 indicated that the sample is brighter (L*= 72.63), redder (a* = 4.12), and more yellow (b* = 35.28). It is recommended the use of 4g of powder to get excellent results.
Table 4: Dyeing of cotton fabric with rose petals obtained from valuable powder amount
| Powder | L* | a* | b* |
| 2g | 73.21 | 3.92 | 32.37 |
| 4g | 72.63 | 4.12 | 35.28 |
| 6g | 72.15 | 3.43 | 34.67 |
| 8g | 70.87 | 5.53 | 34.29 |
| 10g | 68.94 | 6.02 | 35.34 |
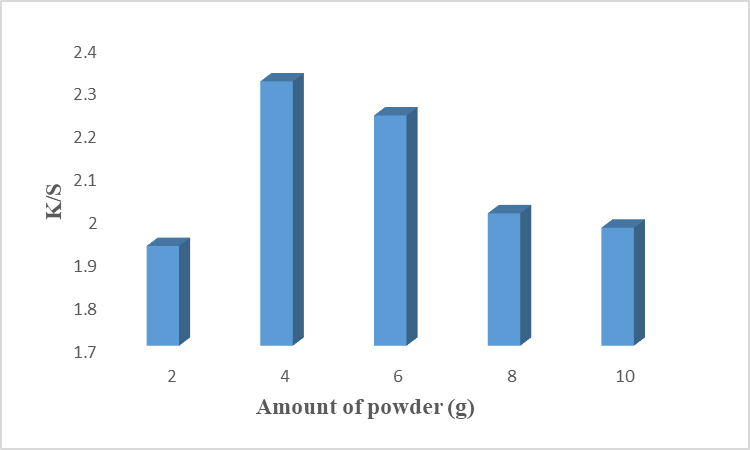
Fig. 4: Effect of different amounts of powder on dyeing of cotton fabrics using cotton fiber
Dyeing Temperature Optimization
In the irradiated cotton dyeing process, heat is an essential and key parameter. It is necessary to find the optimal temperature for the dyeing process. A low heating level does not increase the kinetic energy of the dye molecules to move toward the fiber. While high heating disturbs the equilibrium between dye and fiber and also causes the desorption of dye on the fiber [17]. Optimal heating is necessary for maximum extraction, coloration, and fixation of dye onto the fiber and provides high color strength with a higher K/S value. Fig. 5 shows the effects of dying temperature on the color yield of the dyed cotton fabrics. It is found that irradiation cotton fiber shows excellent color strength at 80o C with the highest K/S value. As cotton fiber is a polymeric substrate in nature, it requires a higher temperature to sorb maximum colorant. Above this temperature, the color strength becomes low. Because higher temperature level causes the degradation of colorant. Lab values in Table 5 revealed that the sample is brighter (L* = 76.92), less red (a* = 1.03), and yellow (b* = 15.98) at 80oC. It is recommended to use 80oC dyeing temperature for maximum color strength.
Table 5: Dyeing of cotton fabric with rose petals at different temperatures
| Temperature | L* | a* | b* |
| 20°C | 78.44 | 0.73 | 15.37 |
| 40°C | 80.12 | 1.26 | 17.29 |
| 60°C | 77.37 | 1.12 | 15.93 |
| 80°C | 76.92 | 1.72 | 17.83 |
| 90°C | 78.54 | 1.03 | 15.98 |
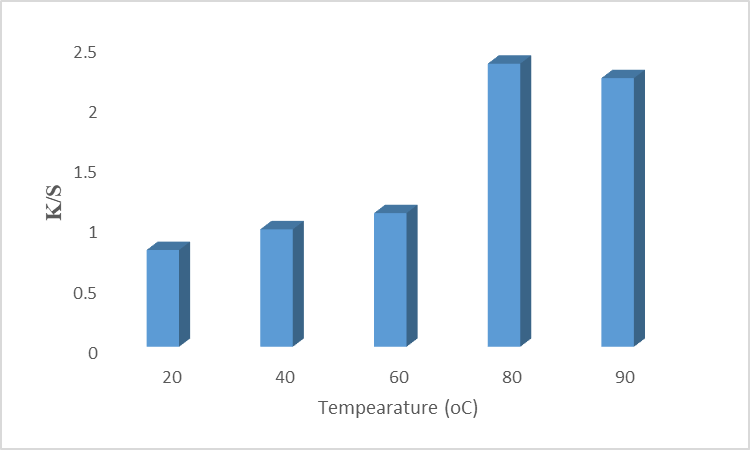
Fig. 5: Effect of temperature on dyeing of cotton fabrics using cotton fiber.
Optimization of Dyeing Time
The rate of the dying process depends on the rate of two factors, diffusion, and fixation of colorant on the fiber. Both factors depend on the contact time between the colorant and fiber. Therefore, time is an important parameter in the dying process and it is important to find the optimal contact time to get the excellent color strength. Low contact time does not accelerate the kinetic energy of the colorant and disturbs the diffusion and even distribution of color on the fiber. While high contact time disturbs the equilibrium of the dye bath which causes the desorption of the colorant [17]. Fig 6 revealed that irradiated cotton fiber gives maximum color strength for 30 minutes with a higher K/S value. Decreasing values were observed with increasing dyeing time which may be due to the over-heating and degradation of the colorant. Lab values show that the sample is brighter (L* = 75.32), less red (a* = 1.39), and yellow (b* = 18.72). It is recommended to precede the dyeing process for 30 minutes.
Table 6: Dyeing of cotton fabric with rose petals at different time
| Time | L* | a* | b* |
| 20 min | 78.93 | 0.67 | 16.23 |
| 30 min | 75.32 | 1.39 | 18.79 |
| 40 min | 77.61 | 1.02 | 9.92 |
| 50 min | 76.82 | 1.27 | 11.02 |
| 60 min | 76.08 | 0.89 | 16.83 |
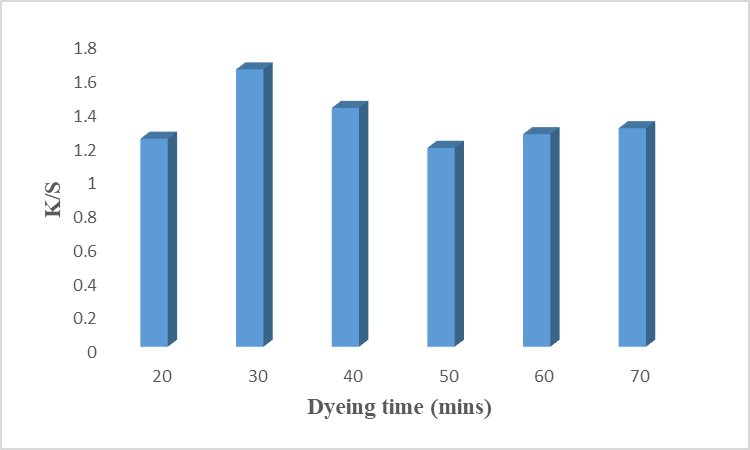
Fig. 6: Effect of time on dyeing of cotton fabrics using rose petals.
Optimization of pH of dyeing bath
Another important factor for the dyeing process is the nature of the medium (pH) of the dyeing bath because only a valid pH level provides excellent color strength. The nature of the dye bath affects the interaction of the colorant with the active site of fiber. It was found that the nature of the dye bath should be acidic for the dyeing of cotton fiber because it protonated NH2+2 due to the presence of H+ which makes the availability for the good interaction with the surface of cotton fiber [18]. Too much acidic medium adds extra H+ while too much alkaline may swell the fiber which weakens the interactions. Our results given in Fig. 7 revealed that fiber shows maximum color strength at 2 pH with a high K/S value. Decreasing values were observed with increasing pH due to the weakening of binding. At high pH, ammonium ion (-NH3+) shifts to the amino (-NH2) group which causes electrostatic repulsion and decreases dye uptake. The colorant is acidic and degraded at higher pH [19]. A previous study showed that 2pH for the dye bath has given excellent results in wool [20]. Table 7 represents Lab characteristics which revealed that the sample is brighter (L* = 75.930), less red (a* = 1.42), and more yellow (b* = 25.24) at 2pH. Hence, it is recommended to dye the cotton fiber with rose petals colorant at 2pH to get excellent results.
Table 7: Dyeing of cotton fabric with rose petals at different pH
| pH | L* | a* | b* |
| 1 | 75.13 | 2.37 | 26.02 |
| 2 | 75.93 | 1.42 | 25.24 |
| 3 | 76.17 | 2.09 | 26.17 |
| 4 | 77.39 | 0.73 | 22.53 |
| 5 | 82.57 | -0.29 | 16.29 |
| 6 | 83.19 | -0.57 | 16.21 |
| 7 | 82.02 | 0.49 | 15.92 |
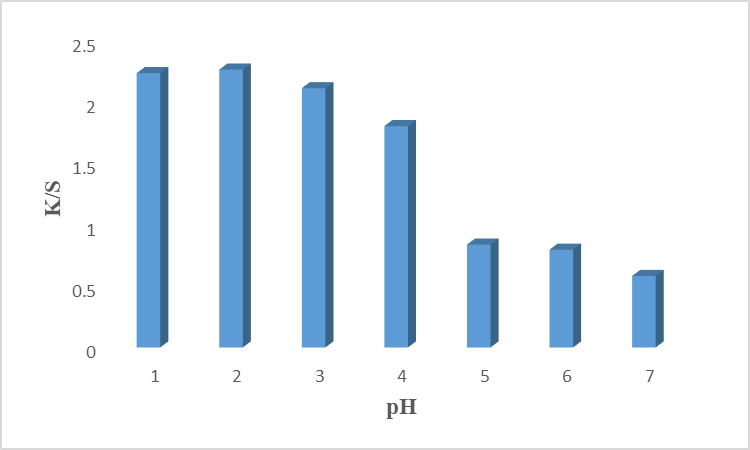
Fig. 7: Effect of pH on dyeing of cotton fabrics using cotton fiber
Salt Concentration Optimization
The salt (NaCl) acts as an exhausting or leveling agent in the natural or synthetic dyeing process. It helps to rush the color from the dye bath to the fabric surface within a range of short attractive forces. The inclusion of salts reduces the repulsion between the negative charge of fabric and dye and develops attractive forces to give maximum color strength [20]. A low amount of salt does not exhaust well to help the colorant to reach the fabric. While the high amount resulted in over-exhausting which cause the aggregation and poor fixation of colorant on the fiber [21]. Our results given in Fig.8 revealed that 3% of NaCl has given maximum exhaustion which results in high color depth with high K/S value. It is found that little amount of salt is required to dye the cotton fiber. While K/S decreases with increasing amounts of salt. This is due to over-exhaustion and poor fixation of colorant. Lab values showed that the sample is brighter (L* = 75.93), redder (a* = 3.27), and more yellow (b* = 32.17). Hence, it is recommended to use 3% of salt as an exhausting agent to get excellent dyeing results on cotton fiber.
Table 8:Dyeing of cotton fabric with rose petals at different amounts of salt NaCl concentration
| NaCl | L* | a* | b* |
| 1 | 74.13 | 4.43 | 32.45 |
| 3 | 75.93 | 3.27 | 32.17 |
| 5 | 72.30 | 4.86 | 37.64 |
| 7 | 75.11 | 3.67 | 34.67 |
| 9 | 73.42 | 4.03 | 36.09 |
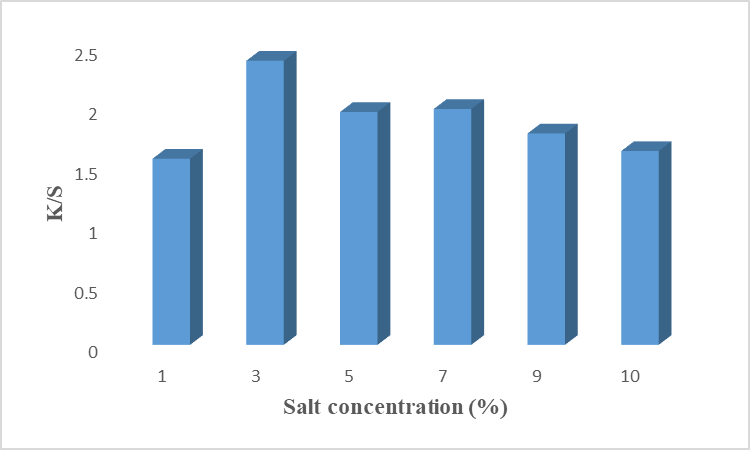
Fig. 8: Effect of salt Conc. (NaCl) on dyeing of cotton fabrics using rose petals
The result is given in Fig 9(a), (b) for chemical mordant, and Fig 10(a), (b) for a bio-mordant show that the strength and fastness properties of color have been improved by mordant. The chemical mordant has shown good color strength and dark shades, this is due to the coordinated covalent interaction of aluminum and iron dye complex with unirradiated cotton fabrics before and after dying [22]. In Fig 9(a) it is found that 5% alum as pre-mordant gives excellent results on cotton fiber with higher K/S value. Similarly, 7% of alum has given good results when used as a post-mordant. Positively charged aluminum ions of alum help in more dye fixation by binding with negatively charged molecules of dye and fiber. Hence, alum forms a complex with dye and fiber with maximum strength at 5% and 7% of alum as pre- and post-mordant, respectively. Results given in Fig. 9(b) revealed that 3% of iron as pre-mordant has given good color strength with a high K/S value. However, 7% of Fe showed a high K/S value when used after the dyeing process. It is necessary to find the optimal condition because a stable metal-dye complex is formed at optimal conditions. Hence, Fe as a chemical mordant form a stable complex at 5% and 7% concentrations when used as pre-mordant and post-mordant, respectively. Similarly, the results for bio-mordants improve the colorfastness properties of the dye onto fiber. This is due to the presence of the –OH group, benzene, and conjugated system which form interactions more firmly [20]. A previous study showed that bio-mordants show high K/S values as well as new shades [23]. In the present study, henna and turmeric have been used as bio-mordants. Results given in Fig. 10(a) show that 1% of henna as pre-mordant has given excellent color properties with higher K/S values. In post-mordanting, 7% of henna gives excellent color strength to the fiber. At these concentrations, the active colorant forms firm bindings with dye and fiber. Different color coordinates are given in Table. 10 shows that most samples of pre- and post-mordanting are brighter, less red, and more yellow. Hence, using henna as a bio-mordant gives excellent results when used before and after the dyeing process. The main coloring agent of turmeric is curcumin which forms a coordination complex with fiber and dye to give yellow color to fiber [24]. Results given in Fig.10(b) show that 5% of turmeric gives good color shade and color strength when applied before the dyeing. In post-mordanting, 7% of turmeric gives desired properties. At optimum concentration, the active colorant of turmeric was firmly bound with anthocyanin and cotton fiber which resulted in good color strength. More than optimum concentration causes precipitation formation which resulted in low color strength. In this study, the effects of different chemical mordants and bio-mordants on the dyeing of cotton fiber using microwave-irradiated acidic extract of rose petals. It is found that bio-mordants, henna, and turmeric, show high K/S values, bright yellow shades, and excellent fastness properties as compared to chemical mordants. This is because other impurities were also added with chemical mordant which resulted in low color shades. The –OH group of bio-mordant forms the interactions with the –OH group of colorant and amide linkage of cotton fiber which resulted in excellent shades and high color strengths. Overall, turmeric gave excellent color strength when used before the dying process.
Table 9(a). Effect of Aluminum Mordant on dyeing of Cotton using rose petals extract
| Mordant concentration | Pre- mordanting | Post-mordanting | ||||
| L* | a* | b* | L* | a* | b* | |
| 1 | 77.23 | -7.02 | 41.92 | 86.35 | -2.63 | 10.37 |
| 3 | 76.97 | -6.13 | 38.18 | 86.14 | -0.93 | 6.17 |
| 5 | 75.14 | -5.21 | 40.86 | 86.02 | -1.04 | 6.64 |
| 7 | 79.08 | -7.38 | 38.52 | 86.56 | -2.55 | 10.56 |
| 9 | 77.55 | -6.38 | 38.86 | 86.93 | -1.71 | 8.02 |
| 10 | 78.12 | -6.99 | 39.12 | 86.77 | -1.59 | 7.88 |
L*=bright a*=red b*=yellow
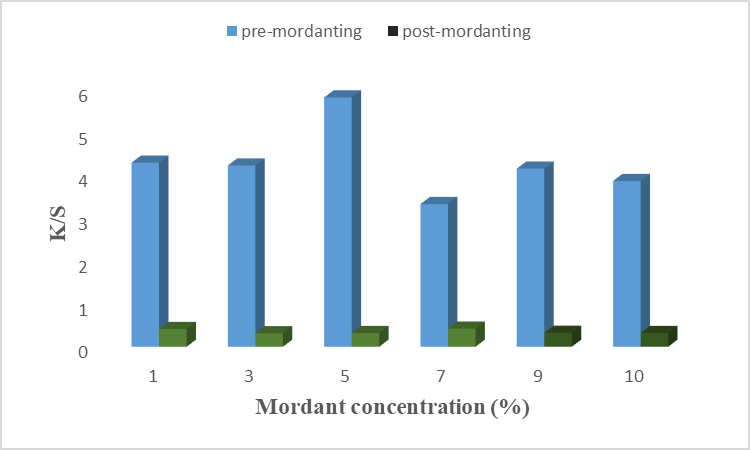
Fig. 9(a). Effect of Aluminium mordant on dyeing of cotton using rose cotton extract
Table 9 (b): Effect of Iron Mordant on dyeing of Cotton using rose petals extract
| Mordant concentration | Pre- mordanting | Post-mordanting | ||||
| L* | a* | b* | L* | a* | b* | |
| 1 | 72.09 | 5.63 | 13.91 | 77.98 | 2.24 | 14.49 |
| 3 | 71.54 | 6.02 | 14.04 | 77.29 | 2.13 | 13.67 |
| 5 | 72.44 | 5.77 | 14.21 | 77.04 | 2.69 | 13.60 |
| 7 | 72.83 | 5.43 | 13.57 | 73.97 | 2.91 | 15.91 |
| 9 | 71.98 | 5.62 | 13.14 | 77.63 | 2.52 | 13.52 |
| 10 | 72.35 | 5.02 | 13.66 | 76.83 | 2.23 | 13.87 |
L*=bright a*=red b*=yellow
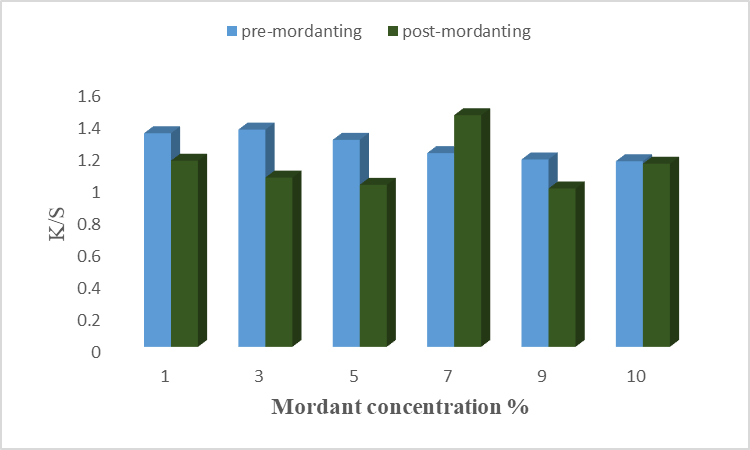
Fig. 9 (b): Effect of Iron mordant on dyeing of cotton using rose petals extract.
Table 10(a): Effect of henna mordant on dyeing of cotton using rose petals extract.
| Mordant concentration | Pre- mordanting | Post-mordanting | ||||
| L* | a* | b* | L* | a* | b* | |
| 1 | 73.66 | 0.98 | 18.64 | 79.96 | -0.31 | 15.91 |
| 3 | 74.59 | 0.83 | 18.57 | 79.53 | -0.19 | 15.96 |
| 5 | 75.05 | 0.84 | 18.24 | 79.39 | 0.49 | 14.62 |
| 7 | 75.39 | 0.86 | 17.67 | 78.98 | 0.12 | 15.21 |
| 9 | 74.43 | 0.79 | 17.98 | 78.84 | 0.18 | 15.02 |
| 10 | 75.43 | 0.49 | 18.42 | 78.91 | 0.52 | 14.76 |
L*=bright a*=red b*=yellow
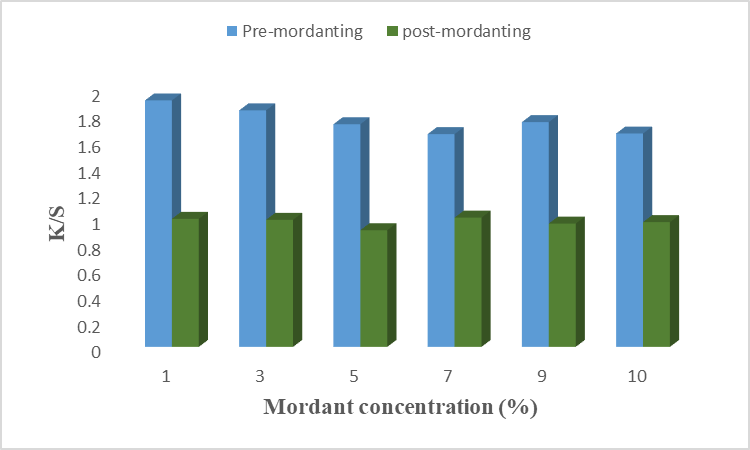
Fig. 10 (a): Effect of henna mordant on dyeing of cotton using rose petals extract.
Table 10 (b): Effect of turmeric mordant on dyeing of cotton using rose petals extract
| Mordant concentration | Pre- mordanting | Post-mordanting | ||||
| L* | a* | b* | L* | a* | b* | |
| 1 | 73.27 | -3.03 | 52.15 | 77.56 | -3.54 | 41.86 |
| 3 | 75.12 | -3.47 | 54.21 | 77.73 | -3.47 | 40.29 |
| 5 | 72.83 | 1.04 | 60.71 | 77.46 | -3.57 | 40.63 |
| 7 | 74.14 | -2.61 | 53.78 | 77.92 | -3.63 | 41.54 |
| 9 | 75.29 | -3.53 | 52.54 | 78.04 | -3.72 | 41.18 |
| 10 | 75.18 | -3.41 | 52.71 | 77.13 | -3.75 | 54.29 |
L*=bright a*=red b*=yellow
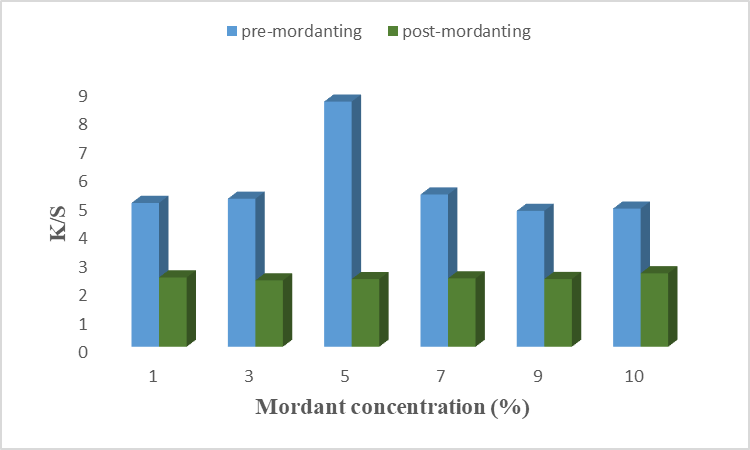
Figure 10 (b): Effects of turmeric mordant on dyeing of cotton fiber using rose petals extract
Conclusion
This study was based on the extraction of natural colorants from rose petals (Rosa indica). Microwave-assisted extraction and dyeing of natural fabrics have promising effects on the coloration of natural fabrics. It is examined that the extract obtained from 4g of rose petal powder followed by microwave treatment for 4 minutes in the acidified methanolic medium has given good results in unirradiated cotton fabrics. The acceptable characteristics have been obtained by dyeing optimal fabric at 85 °C for 45 min of 7 g/L of Glauber’s salt using 25 mL of irradiated dye bath of pH 9. The addition of bio-mordant has made the process more eco-friendly and sustainable, whereas in comparison bio-mordants overall have shown great impact than chemical mordants. It is concluded that microwave treatment has great potential to explore new dye-yielding plants for textile dyeing thereby making the dyeing process more cost, time, and energy effective.
Acknowledgment
This research study is extracted from the masters thesis of 1st author of this article and submitted to Higher Education Commission, Pakistan
References
1. Lu, B., et al., Phytochemical content, health benefits, and toxicology of common edible flowers: a review (2000–2015). 2016. p. S130-S148.
2. Sridevi, V., P. Giridhar, and G.J.G.J.o.M.R. Ravishankar, Evaluation of Roasting and Brewing effect on Antinutritional Diterpenes-Cafestol and Kahweol in Coffee. 2011. 11(5): p. 1-7.
3. Hummer, K.E., J.J.G. Janick, and g.o. Rosaceae, Rosaceae: taxonomy, economic importance, genomics. 2009: p. 1-17.
4. Mansour, H.F., S.J.C.T. Heffernan, and E. Policy, Environmental aspects on dyeing silk fabric with sticta coronata lichen using ultrasonic energy and mild mordants. 2011. 13: p. 207-213.
5. Prabhu, K. and M.J.J.o.S.C.S. Teli, Eco-dyeing using Tamarindus indica L. seed coat tannin as a natural mordant for textiles with antibacterial activity. 2014. 18(6): p. 864-872.
6. Rather, L.J., et al., Ecological dyeing of woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. 2016. 4(3): p. 3041-3049.
7. Sinha, K., et al., Extraction of natural dye from petals of Flame of forest (Butea monosperma) flower: Process optimization using response surface methodology (RSM). 2012. 94(2): p. 212-216.
8. Ebrahimi, I. and M.J.C.T. Parvinzadeh Gashti, Extraction of polyphenolic dyes from henna, pomegranate rind, and Pterocarya fraxinifolia for nylon 6 dyeing. 2016. 132(2): p. 162-176.
9. Khattak, S.P., et al., Optimization of fastness and tensile properties of cotton fabric dyed with natural extracts of Marigold flower (Tagetes erecta) by pad-steam method. 2014. 11(7s): p. 52-60.
10. Chen, Y., M.-Y. Xie, and X.-F.J.J.o.f.e. Gong, Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. 2007. 81(1): p. 162-170.
11. Galan, A.-M., et al., New insights into the role of selective and volumetric heating during microwave extraction: Investigation of the extraction of polyphenolic compounds from sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. 2017. 116: p. 29-39.
12. Ekezie, F.-G.C., et al., Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. 2017. 67: p. 160-172.
13. Saxena, S., et al., Natural dyes: sources, chemistry, application and sustainability issues. 2014: p. 37-80.
14. Adeel, S., et al., Appraisal of marigold flower based lutein as natural colourant for textile dyeing under the influence of gamma radiations. 2017. 130: p. 35-39.
15. Adeel, S., M. Zuber, and K.M. Zia, Microwave-assisted extraction and dyeing of chemical and bio-mordanted cotton fabric using harmal seeds as a source of natural dye. Environmental Science and Pollution Research, 2018. 25(11): p. 11100-11110.
16. Shabbir, M., et al., An eco-friendly dyeing of woolen yarn by Terminalia chebula extract with evaluations of kinetic and adsorption characteristics. 2016. 7(3): p. 473-482.
17. Adeel, S., et al., Microwave-assisted eco-dyeing of bio mordanted silk fabric using cinnamon bark (Cinnamomum Verum) based yellow natural dye. Sustainable Chemistry and Pharmacy, 2020. 17: p. 100306.
18. Bhuiyan, R., et al., Coloration of polyester fiber with natural dye henna (Lawsonia inermis L.) without using mordant: a new approach towards a cleaner production. 2018. 5(1): p. 1-11.
19. Singhee, D. and P.J.J.o.N.F. Samanta, Studies on dyeing process variables for application of Tesu (Butea monosperma) as natural dye on silk fabric. 2019. 16(8): p. 1098-1112.
20. Adeel, S., et al., Microwave-assisted eco-dyeing of bio mordanted silk fabric using cinnamon bark (Cinnamomum Verum) based yellow natural dye. 2020. 17: p. 100306.
21. Adeel, S., et al., Sustainable Isolation and Application of Rose Petals Based Anthocyanin Natural Dye for Coloration of Bio-Mordanted Wool Fabric: Short title: Dyeing of Bio Mordanted Wool With Rose Petal Extract. 2022. 19(13): p. 6089-6103.
22. Bouatay, F., et al., Dyeing behavior of the cellulosic and jute fibers with cationic dyes: process development and optimization using statistical analysis. 2016. 13(4): p. 423-436.
23. Batool, F., et al., Sustainable Dyeing of Cotton Fabric Using Black Carrot (Daucus carota L.) Plant Residue as a Source of Natural Colorant. 2019. 28(5).
24. Hussaan, M., et al., Microwave-assisted enhancement of milkweed (Calotropis procera L.) leaves as an eco-friendly source of natural colorants for textile. 2017. 24: p. 5089-5094.

